Presentation on the topic: Applying batteries. Presentation "Automotive Batteries" Presentation on the use of batteries in physics

The battery is designed to supply major consumers of the car in the parking lots, in emergency modes and at low speeds of the train. The main consumers of the car, the chains of alarm, protection and control can be powered by the battery not only at stops, but also with a sudden output of the generator failure during movement. In addition, the battery performs a protective function: it reduces the magnitude of the switching overvoltages arising from the disconnection of consumers during the generator operation. The battery also makes it possible to control the operation of basic consumers, control circuits, protection devices, and alarm during inspections of the receiving wagons before departing to the flight and upon arrival from it. Rechargeable batteries are placed under a wagon in special boxes equipped with ventilation to remove an explosive mixture formed when charging the battery.

On wagons without air conditioning with the rated voltage of the electrical network 50 V, you set batteries consisting of 26 acid or alkaline batteries. On wagons with air conditioning installations with a rated voltage of an electrical network 110 V, batteries consisting of 56 acid or alkaline batteries are installed.



The batteries during charge are isolated hydrogen and oxygen, which at a certain concentration form an explosive mixture (random gas). Its content in the air over 9% is considered explosive. Therefore, the civagned battery packs 5 are equipped with ventilation, which consists of fence blinds 6 at the bottom of the battery box and deflectors / (knee-like pipes) located on the side wall of the drawer or on its lid. Ventilation is carried out as a result of air supply through intating blinds due to the resolution occurring around the deflector head when the train is moving. In order to avoid contamination of the inner surface of the ventilation box of the hole in the intake Lui, they are made in the form of a labyrinth. For the battery-powered batteries used on the charging current cars, about 60 and the volume of fresh air for ventilation should be m3 / h. In some wagons to enhance the air exchange in sub-breasted battery boxes during the charge of batteries in the parking lots, a system of forced ventilation is provided. It consists of an electric fan, which turns on automatically when the electric motor is started, leading to the rotation of the carriage generator in the parking lots in order to charge the battery.

Principle of operation of an acid battery. In the charged battery, the active mass of the positive plates consists of PBO2 lead dioxide, negative-of spongy PB lead. The plates are immersed in an electroly-aqueous solution of sulfuric acid, the density of which, depending on the time of year, the operation of the battery and its type can vary in the range of 1.22- 1.28g / cm 3

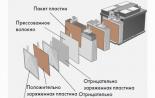
Acid Battery Prism Prism Space Space Space Space Negative Plates Negative Plates Tube Tube League Rod Lead Tube Separator Separator Positive Plates Positive Plates Safety Machine Safety Motor Bridge Positive Plates Bridge Positive Plates Output Positive Plate Plates Plug Positive Plates Ebonite Tank Ebonite Tank Cover Cover Rubber Ring Rubber Ring Cork Cork Nut Nut Pointer Electric Pointer Electric Tank Plug Negative Plates Relinted Metal Plates Bridge Metal Plates Bridge Negative Plates Metal Pottone Metal Pottone Shock Absorbers Shock Absorbers Positive Clamp Positive Clement Copper Tire Copper Tire Wooden Box Wooden Box Positive Bus Positive Box Tire negative tire negative tire negative clamp denied Clamp clamp clip clip handle carrying handle carrying






Alkaline batteries have a large mechanical strength, do not fail as a result of low temperatures, have a long service life, do not require such careful care as acid. As a result, alkaline batteries get more distribution. However, the main alkaline batteries are low efficiency and their significant internal resistance.

Alkaline batteries with lamella plates are installed on passenger cars, which are assembled from special boxes made of nickel-plated steel tape. Lamella, filled with active mass, interconnected into the lock and bonded with each other from two sides by the Ribs, to which the contact bar is welded. As a result, durable insecuring design is formed. For free access of the electrolyte to the active mass in the walls of the lamella, there are a large amount of fine holes with a small diameter, so the active mass is not poured. The active mass of the positive plates of alkaline batteries is mainly made of nickel oxide hydrate, to which they are added to increase the electrical conductivity of graphite and the active addition of barium oxide hydrate. The active mass of the negative plates of the nickel-iron battery consists of powder iron and its oxides with the addition of a small amount of nickel sulfate and sulfur iron. Alkaline batteries with lamella plates are installed on passenger cars, which are assembled from special boxes made of nickel-plated steel tape. Lamella, filled with active mass, interconnected into the lock and bonded with each other from two sides by the Ribs, to which the contact bar is welded. As a result, durable insecuring design is formed. For free access of the electrolyte to the active mass in the walls of the lamella, there are a large amount of fine holes with a small diameter, so the active mass is not poured. The active mass of the positive plates of alkaline batteries is mainly made of nickel oxide hydrate, to which they are added to increase the electrical conductivity of graphite and the active addition of barium oxide hydrate. The active mass of the negative plates of the nickel-iron battery consists of powder iron and its oxides with the addition of a small amount of nickel sulfate and sulfur iron.

Alkaline Battery Case Case Case Case Rubber Case Rubber Negative Semi-Block Negative Semi-Block Bay Hole Hole Bay Hole Cover Cover Cover Pulp Pulp Pulp Separators Separators Positive semi-block Positive semi-block

The discharge and charge of an alkaline battery when the alkali battery discharge hydrate Ni-niche (OH) 3 in the positive electrode, interacting with the electrolyte ions, is moving into nickel nickel hydrate (OH) 2, and the iron or cadmium of the negative electrode turns into a hydraulic oxide hydrate Fe ( OH) 2 or CD (OH) 2 cadmium oxide hydrate. In the process of electrochemical reactions arising from this, the chemical energy goes into electrical and between the electrodes there is a difference in potentials about 1.5 V, which ensures the flow of current on the outer chain and inside the battery. When the alkali battery is discharged, the nickel nickel (OH) 3 in the positive electrode, interacting with the electrolyte ions, goes into the nickel nickel hydraulic hydrate (OH) 2, and the iron or cadmium of the negative electrode turns into a hydraulic oxide hydrate FE (OH) 2 or hydrate Oxide Cadmium CD (OH) 2. In the process of electrochemical reactions arising from this, the chemical energy goes into electrical and between the electrodes there is a difference in potentials about 1.5 V, which ensures the flow of current on the outer chain and inside the battery. The electrolyte in the process of electrochemical reactions is not consumed, so it does not change its density during the operation of the alkaline battery. When charging the battery under the action of electrical energy supplied from an external current source, the active mass of the positive plates occurs, accompanied by the transition of nickel nickel nickel (OH) 2 in nickel hydrate Ni (OH) 3 hydrate. At the same time, the active mass of negative plates is restored to the formation of sponge iron FE or CD spongy cadmium. To fully use the tank of the negative electrode, the positive electrode must have twice the active mass. Machine batteries, as a rule, to recharge better than it is not possible, since deep discharges and incomplete charges contribute to premature way out of order. The temperature increase over 45 ° also leads to rapid destruction of the active mass of the electrodes.


Alkaline battery device. In an alkaline battery, the active mass of the positive electrode consists of hydrate oxide hydrate Ni (OH) 3, and the active mass of the negative electrode from sponge iron Fe (iron-nickel batteries) or from the mixture of CD spongy cadmium and spongy FE (cadmium-nickel batteries). An electrolyte is a 20% solution of caustic cavity con with an admixture of caustic lithium. This impurity significantly increases the battery life. Iron-nickel batteries manufactured by the domestic industry have the designation of the LG, the cadmium-nickel KN. Both electrodes in these batteries are made in the form of steel nickel-plated lattices, in the cells of which were known to be filled with active weight of the box (lamella) from nickel-plated tin with a large number of fine holes for accessing the electrolyte to the active mass. Each negative plate is located between two positive; To prevent a short circuit between them, separators are installed in the form of ebonite rods. The vessel in which the plates and electrolyte are placed is also made of nickel-plated tin and has a welded cover with holes for output conductive pins and to exit gases and pouring electrolyte. To make the vessel of the mechanical strength of the wall, it is performed corrugated.

Alkaline batteries with lamella plates are installed on passenger cars, which are assembled from special boxes made of nickel-plated steel tape. Lamed, filled with active mass, interconnected into the lock and fasten with each other from two sides by Rybra, to which the contact bar is welded. Alkaline batteries with lamella plates are installed on passenger cars, which are assembled from special boxes made of nickel-plated steel tape. Lamed, filled with active mass, interconnected into the lock and fasten with each other from two sides by Rybra, to which the contact bar is welded.





Installation of batteries batteries batteries are mounted in special boxes that are attached under the car body. These boxes are made of sheet steel, painted with acid-resistant paint and have folding covers with guides for which batteries can be pulled out when replacing, examining or tightening the electrolyte. The covers are sealed with shaped rubber gaskets. Acid batteries in most cases are installed in a camp battery drawer in one row. Longitudinal movements of batteries are prevented by wooden spacer bars. Wooden stubborn bars on batteries, resting in the lid when closing a camp battery box, protect batteries from transverse movements. To increase the resistance of the battery isolation and reduce the leakage current, the batteries are installed on the insulators, and the gap is formed between the bottom of the box and the battery. On foreign buildings, the batteries are installed on oblong ceramic angular insulators, which simultaneously facilitate the extension of the batteries from the inspection and maintenance box. On the box of the box, the fuse of the battery closed with a casing is installed. To determine the state of the battery during receiving the wagons before the flight, the head, the train electromechanics and the conductor should know which type of batteries is installed on the received wagons. A sign of battery charges is the constant value of its voltage after turning on the load. The voltage drop below the minimum permissible indicates that the battery is discharged. In this case, it must be charged or replaced. The electrolyte must fill out a bank not lower than 50 mm and not higher than 65 mm relative to the top edge of the plates. Before checking, you need to turn off all energy users. During the flight, check the ammeter when the generator mode is turned off. If the generator works correctly, the arrow of the ammeter deviates depending on the connected consumers. If the arrow remains in position 0, this should be informed by the train head to prevent a strong battery discharge. If the battery was discharged with a long parking lot or was not enough charged due to the low speed of movement, the battery from the foreign direct current source should be charged. Rechargeable batteries should be stored technically sound, in chargeable state, with removed fuses. Before sending wagons to sucks, batteries are examined, purified from salts, dust, dirt, snow, dryly rub, if necessary, neutralize the surface of each battery, check the level and density of the electrolyte, adjust it, measure the voltage of each battery with a load fork with resistance corresponding to the current 5 - Summer discharge of batteries. Revealed when checking "remaining" batteries, as well as having an internal break, short-circuited or ignited replace the equivalent to most battery batteries. When replacing batteries batteries charge, after which each battery is checked with a load fork. Acid batteries in the remote need to recharge monthly.

Only technically serviceable normally charged batteries are installed on the car, which must be securely secured. By security and sanitation conditions, they are placed in special battery boxes that are under the body of the car. Boxes and racks should be clean and dry. It is necessary to firmly fix the tips of the intercoccuable connections, since with a loose contact may occur. After installing and checking the absorption resistance of the battery with respect to the car body, all bores of batteries, jumpers, nuts are covered with a thin layer of vaseline. In case of inspection and repair of batteries, it is necessary to observe special caution due to the fact that the batteries during charging are excreted hydrogen and oxygen, which at a certain concentration form an explosive mixture. It is strictly forbidden to inspect the batteries with open fire, as well as detect faulty batteries by closing their output clamps with metal objects, which leads to the formation of sparks.


Slide 1.
 Slide 2.
Slide 2.
 Slide 3.
Slide 3.
 Slide 4.
Slide 4.
 Slide 5.
Slide 5.
 Slide 6.
Slide 6.
 Slide 7.
Slide 7.
 Slide 8.
Slide 8.
Presentation on the topic "Application of batteries" can be downloaded absolutely free on our website. Project subject: Physics. Colorful slides and illustrations will help you interest your classmates or audience. Use the player to view the contents, or if you want to download the report - click on the appropriate text below the player. Presentation contains 8 slide (s).
Slides presentation
https://cloud.prezentacii.org/15/04/40675/images/thumbs/screen3.jpg "Alt \u003d" (! lang: battery. - This is an electric current source, the action of which is based on chemical reactions. Unlike the usual The battery galvanic element can be charged and discharge a large number of times. The ability to accumulate charge and the ability to recharge batteries in a separate class" title="Battery. - This is an electric current source, whose action is based on chemical reactions. Unlike the conventional electroplating element, the battery can be charged and discharge a large number of times. The ability to accumulate charge and the ability to recharge batteries in a separate class">!}
Slide 3.
Battery
This is an electric current source, the action of which is based on chemical reactions. Unlike the conventional electroplating element, the battery can be charged and discharge a large number of times. The ability to accumulate the charge and the ability to recharge batteries into a separate class of devices, widely used both in production and in everyday life.

Slide 4.
The last years of the twentieth century are the years of the wide distribution of such portable devices, like players, pagers, cell phones, various portable computers, etc. As a source for them, it is not only convenient to use batteries, but also it is impossible to use anything else. Despite some differences, all batteries for portable electronic devices are inherent in many common properties: a large capacity (the battery must work for a long time without recharging), small size and weight (person using this device must be easily and convenient to carry it), high reliability ( The batteries should not be susceptible to various shocks, shakements, temperature drops, etc.). All these requirements are most satisfied with lithium-metal hydride batteries.

Slide 5.

Slide 6.
If earlier the computer was a tool for scientists, then he was currently published in everyday life and in business. In the latter case, an important data may be lost with a sudden disconnection of electricity, which will lead to serious losses. If this happens with a large server, then the consequences can even be disastrous. So that this does not happen, use the uninterruptible power supply (UPS) source, the most important element of which is the battery. The requirements for it are several others than to the battery for portable devices. The battery must work for a long time without recharging and should give voltage enough to normal operation of the computer at its outputs. For it, sometimes the output power is 500 W and more.

Slide 7.
In addition to the wide propagation of batteries in the above devices, the main use of the battery has found in the automotive industry. In cars, it is used for the initial start of the engine. Despite the general lower indicators of the latter compared to lithium metal-hydride, it is lead batteries that use lead batteries due to ease of use, relative cheapness and simply automotive traditions.

To enjoy previewing presentations, create yourself an account (account) Google and log in to it: https://accounts.google.com
Signatures for slides:
Pupils 8 "B" MOU SOSH No. 38 DROFICHEY ANASTASY BATTER - the device for the accumulation of electrical energy in order to further use it.
Italian scientist Luigi Galvani (1737-1798) opened the possibility of obtaining electric current other than electrification by friction of batteries
Once, when he conducted a study of frogs, he noticed that when touched a steel scalpel to the nerve, the paw was a dead frog in motion. In the future, Galvani put several experiments to detect the cause of the occurrence of electric current and translas
The principle of batteries is based on the electrolysis phenomenon. Electrolysis is a change in the chemical composition of the solution when the electric current is passed through it, due to the loss or addition of electrons by ions. An important property of electrolysis is its reversibility. E lecturer
Similar to the galvanic element, you can make a battery. To do this, use two lead plates immersed in a solution containing one part of sulfuric acid and five parts of water. To charge the battery, it is connected in series with an ammeter and passed through a circuit current. Battery manufacturing
The charging process is that two identical battery plates due to electrolysis become different; One of them, negative, still remains lead, and the material of another (positive) turns into lead peroxide. When the electric current battery passes on the cathode, hydrogen bubbles are released on the cathode, and oxygen is released on the anode. Due to the fact that a certain amount of oxygen is chemically connected to the material of the anode plate, it gradually acquires a dark brown color due to the formation of lead peroxide on its surface. Charging process
When forming the charging current drops, which indicates an increase in the internal resistance of the battery. If the battery is fully charged, the voltmeter attached to it will show a voltage of a few more than 2 volts. Charging Tok
The following chemical reactions occur in the battery (in the process of charging the reaction, go to the left right, when discharged - in the opposite direction): Charging\u003e
Positive plates in the production of industrial batteries are covered with a thick layer of lead peroxide. Negative plates are made of porous spongy lead. In a conventional battery consisting of three successively connected battery elements, the voltage is a little more than 6 volts. The efficiency of the battery is approximately 75%. On the rechargeable battery put a number that shows the number of electricity stored in the battery, expressed in amps - industrial batteries
For example, 120 amps-hours means that with a complete discharge, the battery will be able to give a current of 1 amps for 120 hours, or a current of 2 amps for 60 hours. It is necessary to constantly maintain the battery in the charged state. Even if the battery is not in operation, it should be regularly recharged. It is necessary to contain clips of batteries clean and protected from corrosion. Neither should be allowed to freeze batteries. Ampere - hours
Basically, rechargeable batteries are used to start motor engines and other machines. It is also possible to use as temporary sources of electricity in places remote from settlements. It must be remembered that the batteries should be maintained in the charged state, applying for this, for example, solar energy. In the future, the batteries expect to be used to power eco-friendly electric motors. Application Ampere - hours
On the topic: Methodical development, presentations and abstracts
in this material, the results of the application of the teacher in its work of one of the methods of modern training technologies in extracurricular work are set out - project method ...
Work programs for profession 270802.09 Wizard of general construction work: OP.03 Basics of construction drawing. OPO.04.Onovy technology of general construction works. PM.03 Performing stone works.
Work programs are designed to study by profession 270802.09 Master of general construction work ...
Regulations on graduation qualifications (graduation practical qualifications and written examination)
In this position, the regulatory framework is prescribed to which we rely, the requirements for the design and content of the final qualification work of educational technical school on the direction of the initial profession ...
Continuity in the work of the Defectologist Teacher (Logoped Teacher) and the educator in working with preschoolers with a delay in mental development (from experience)
The effectiveness of the correction of deficiencies in the development in children with the CPR depends on the effective interaction of species operating in the group ....
Slide 1.
"Application of batteries."Clade 2.

Slide 3.
 The battery is an electric current source, which is based on chemical reactions. Unlike the conventional electroplating element, the battery can be charged and discharge a large number of times. The ability to accumulate the charge and the ability to recharge batteries into a separate class of devices, widely used both in production and in everyday life.
The battery is an electric current source, which is based on chemical reactions. Unlike the conventional electroplating element, the battery can be charged and discharge a large number of times. The ability to accumulate the charge and the ability to recharge batteries into a separate class of devices, widely used both in production and in everyday life.
Slide 4.
 The last years of the twentieth century are the years of the wide distribution of such portable devices, like players, pagers, cell phones, various portable computers, etc. As a source for them, it is not only convenient to use batteries, but also it is impossible to use anything else. Despite some differences, all batteries for portable electronic devices are inherent in many common properties: a large capacity (the battery must work for a long time without recharging), small size and weight (person using this device must be easily and convenient to carry it), high reliability ( The batteries should not be susceptible to various shocks, shakements, temperature drops, etc.). All these requirements are most satisfied with lithium-metal hydride batteries.
The last years of the twentieth century are the years of the wide distribution of such portable devices, like players, pagers, cell phones, various portable computers, etc. As a source for them, it is not only convenient to use batteries, but also it is impossible to use anything else. Despite some differences, all batteries for portable electronic devices are inherent in many common properties: a large capacity (the battery must work for a long time without recharging), small size and weight (person using this device must be easily and convenient to carry it), high reliability ( The batteries should not be susceptible to various shocks, shakements, temperature drops, etc.). All these requirements are most satisfied with lithium-metal hydride batteries.
Slide 5.

Slide 6.
 If earlier the computer was a tool for scientists, then he was currently published in everyday life and in business. In the latter case, an important data may be lost with a sudden disconnection of electricity, which will lead to serious losses. If this happens with a large server, then the consequences can even be disastrous. So that this does not happen, use the uninterruptible power supply (UPS) source, the most important element of which is the battery. The requirements for it are several others than to the battery for portable devices. The battery must work for a long time without recharging and should give voltage enough to normal operation of the computer at its outputs. For it, sometimes the output power is 500 W and more.
If earlier the computer was a tool for scientists, then he was currently published in everyday life and in business. In the latter case, an important data may be lost with a sudden disconnection of electricity, which will lead to serious losses. If this happens with a large server, then the consequences can even be disastrous. So that this does not happen, use the uninterruptible power supply (UPS) source, the most important element of which is the battery. The requirements for it are several others than to the battery for portable devices. The battery must work for a long time without recharging and should give voltage enough to normal operation of the computer at its outputs. For it, sometimes the output power is 500 W and more.
Slide 7.
 In addition to the wide propagation of batteries in the above devices, the main use of the battery has found in the automotive industry. In cars, it is used for the initial start of the engine. Despite the general lower indicators of the latter compared to lithium metal-hydride, it is lead batteries that use lead batteries due to ease of use, relative cheapness and simply automotive traditions.
In addition to the wide propagation of batteries in the above devices, the main use of the battery has found in the automotive industry. In cars, it is used for the initial start of the engine. Despite the general lower indicators of the latter compared to lithium metal-hydride, it is lead batteries that use lead batteries due to ease of use, relative cheapness and simply automotive traditions.
Slide 8.
 For quite a long time, humanity is trying to build an electric car, working not on liquid fuel, but on electric current. The main advantage of the electric vehicle compared to the usual car is environmental purity. The source of the current should be large batteries of batteries. It is because of the size of batteries, electric vehicles still did not become serious ruling competitors on gasoline or diesel fuel.
For quite a long time, humanity is trying to build an electric car, working not on liquid fuel, but on electric current. The main advantage of the electric vehicle compared to the usual car is environmental purity. The source of the current should be large batteries of batteries. It is because of the size of batteries, electric vehicles still did not become serious ruling competitors on gasoline or diesel fuel.
"Plasma Physics" - comparing the properties of plasma, gas, solid body. Debayevskaya shielding. Prospects for systems with magnetic retention. Plasma fluctuations. Electric, centrifugal and gradient drift. Aadiabatic invariants. http://sec.gsfc.nasa.gov/. Plasma physics for physicists. -M., Atomizdat, 1979. Crystal. M.1996.
"Using DC" is a system of operational direct current. Areas of use of DC systems (stationary batteries).
"Measurement of current force" - uniform movement movement with uniform motion uneven movement. Type of set. Computer measuring unit. The composition of the set. List of proposed experiments ... Computer measuring unit L-micro connected to a computer or demonstration stopwatch. Optics. Ege-laboratory.
"Electrical resistance grade 8" - Electrical resistance - R. Physician teacher: Gruchutskaya G.Ya. - The interaction of moving electrons with the ions of the crystal lattice. Ust-Tarka secondary school. Cause. Presentation on the topic: "Electrical resistance of conductors". Units of resistance. R \u003d u / i. 1Ω \u003d 1B / a. Different conductors have different resistance.
"Electrical voltage grade 8" - in chains: i1 \u003d i2 but: current operation (a) A1