Technological chrome process
Technological operations when repairing (recovery) parts of chromium are performed in the following sequence.
Mechanical restoration. The surfaces of the parts to be chrome are grinding to the removal of traces of wear and obtain the necessary geometric shape.
Flushing parts in organic solvents and windscreen wrap. Gasoline, kerosene, trichloroethane, benzene, etc. are used as solvents.
Installation of parts on the suspension. It is necessary to monitor the parts equally defended from the anode surface. The bath should be loaded with homogeneous parts reinforced on the same suspension. Pendants and contacts must be made of identical materials. Contact hooks are recommended to be made of bronze and copper. It is used as a material for suspension, steel, the cross sections of the suspensions are calculated, based on the current density of 0.7 ... 1.0 A / mm2. Daily anodes are cleaned of oxides and electrolyte plates.
The temperature of the electrolyte is 60 ... 70 °, current density - 5 ... .15 A / DM2. Exposure time on the cathode - 2 ... 3 min, and on anode - 1 ... 2 min. After degreasing, the details are first washed with hot water (60 ... 80 °), and then cold. Degreasing is considered complete, if, after washing the water is uniformly wets the surface. After degreasing is insulated1 surfaces not subject to chromium. For insulation, you can use perchlorvinyl lacquer, ak-20 varnish, celluloid, viniplast, plexiglas, chlorvinyl tubes or chlorvinyl "insulating tape.
Disciping is the process of processing parts in chromium * electrolyte, consisting of 100 g of chrome anhydride (CZOZ) and 2 ... 3 g of sulfuric acid (H & SO4) on 1 liter of water.
The decaption (etching) of steel parts is carried out for 30 ... 90 s with a current density of 25 ... 40 A / DM2. And for the details of the gray cast iron, the best results, in the sense of clutch strength, are achieved with a current density of 20 ... 25 A / DM2 and duration of decaption 25 ... 30 seconds. The electrolyte temperature in all cases should be 55 ... 60 ° C.
Chrome process. After anodic decapping, the parts are loaded into the chromium bath and warm them off when the current is turned off for 5 ... 6 minutes, and then give a full current according to the chromium mode. When chroming cast-iron parts at first for 3 ... 5 minutes, the "push of the current" is given at a density, 2 ... 2.5 times higher than the selected mode. Electrolyte temperature fluctuations can be within ± 1 ° C. Current breaks are not allowed in the process of electrolysis, as they cause chromium coating. You can continue the process after the current break, if the chromed surface is subjected to anodic etching at a current density of 25 ... 30 A / DM2 for 30 ... 40 s, and then change the current direction. In this case, the deposition of chromium should begin with a cathode current density of 20 ... 25 A / DM2 and gradually increase to normal.
Anodes for chromium are made of pure lead or alloy consisting of 92 ... 93% lead and 7 ... 8% antimony. Anodes made of pure lead are more coated with an insoluble and non-conductive film of chromium-oxidal lead than the anodes from lead alloy and antimony. In most cases, the anodes are made flat and cylindrical. When chromating parts of the complex configuration of the outline of the anode are determined by the form of the cathode. Distance between the anodes and details recommended Make 30 ... 35 mm, but not more than 50 mm. The distance from the bottom of the bath should be at least 100 ... 150 mm, and from the top level of the electrolyte - at least 50 ... 80 mm. The electrolyte level must be below the top edges of the bath per 100 ... 150 mm. When the parts are wrapped in the bath, it is necessary that all the areas of the anodes are equally removed from the opposite sections of the cathode. At the same time, the thickness of the chromium layer is laid uniform over the entire surface of the part.
The depth of immersion of the anodes and parts (cathodes) in the bath should be the same, since at different depths at the edges of the chromed parts are formed thickening, distorting form. The deposition rate of the chromium layer at a current density of 40 ... 100 A / DM2 is 0.03 ... 0.06 mm / h.
Upon completion of the chrome process, the parts are discharged from the bath and together with the suspensions are washed in cold water (in the electrolyte collection) 15 ... 20 s. Finally, the details are washed in cold running water.
Coating processing. Washed and peeled parts are sometimes subjected to heat treatment at a temperature of 150-200 ° C for 2 ... 3 hours, and then mechanical.
Circles are used for grinding soft or medium hardness with grain size from 60 to 120. Grinding is carried out with intensive cooling with liquid and at a circle of 20 ... 30.m / s and above. The speed of rotation of the part-12 ... 20 m / min.
Electrolysis modes. The chromium deposition process and the properties of chromium coatings depend on the mode at which the chrome on the metal surface is precipitated, that is, from the cathode density of the current and the electrolyte temperature. The most clear idea of \u200b\u200bthe approximate boundaries of electrolysis modes that ensure the obtaining of gray, shiny and milk precipitation of chromium gives a current density diagram and temperature (DK-T) shown in Figure 19.
Gray chromium sediment appears on the cathode at low electrolysis temperatures (35 ... 50 ° C) and a wide range of current densities. The precipitation of brilliant * chromium has a high hardness (6000 ... 9000 N / mm2), high wear resistance and smaller fragility.
Fig. 19. Zones of chrome precipitation.
Milk chromium is obtained at higher temperatures, electrolyte (above 70 ° C) and a wide current density interval. Dairy precipitates are characterized by a reduced hardness (4400 ..- 6000 N / mm2), plasticity and increased corrosion resistance.
Porous chrome. Porridge chromium is used in the repair of friction parts in a pair with different metals and alloys at high specific pressures and circumferential speeds or at elevated temperatures. Such details include cylinder sleeves of internal combustion engines, crankshafts, etc.
Porous chromium coatings can be obtained by mechanical, chemical and electrochemical methods.
With a mechanical method on the surface of the part to chromium, recesses in the form of pores or channels. Such preparation is provided by the overall roller, shot blasting and other methods. After chrome, irregularities obtained during preparation are reproduced.
Chemical method is obtained by porosity by etching the surface in hydrochloric acid.
The highest distribution was obtained by an electrochemical method of obtaining porous chromium. This method is the anode processing of chrome-plated parts in the electrolyte of the same composition. Depending on the chromium modes, the porosity of chrome coatings can be two types - the channel and point. When repairing cylinder sleeves, bushings, crankshafts and similar parts, the channel type of porosity is used. Takuk\u003e Porosity and the smallest wear under friction conditions can be obtained when chroming in an electrolyte, consisting of 250 g of CG03 and 2.5 g of H2S04 per 1 liter of water, at an electrolyte temperature |60 + 1 ° C and cathode current density 55 ... 60 A / DM2. The etching is carried out at an anode current density of 35 ... 45 A / DM2 for 8 minutes in the same electrolyte.
The point porosity is formed when chroming in a universal electrolyte at a current density of 45 ... 55 A / DM2 and a temperature of 50 ... 55 ° C. Anodic treatment is carried out in the same way as under the tubular porosity, i.e., at a current density of 35 ... 45 A / DM2 for 8 minutes.
Chrome in self-regulating electrolyte. Recently, a new chromic electrolyte has been developed, called high-speed self-regulating, its composition: chrome 'anhydride - 225 ... 300 g / l, silkmontluoride potassium - 20 g / l and sulphate strontium - 6 g / l.
In such an electrolyte, the current exit during chromium is 17 ... 22%. It is named self-regulating because, with electrolysis, it automatically supports the necessary concentration of the anions administered to the chromium electrolyte. This occurs as a result of an excessive amount of hard-soluble salts of silk silkmist of potassium and sulfate strontium, the solubility of which varies depending on the concentration of chromium anhydride and electrolyte temperature.
To obtain a wear-resistant coating in self-regulating electrolyte, it is recommended to observe the following chromeration modes: current density 50 ... 100 A / DM2, electrolyte temperature 45 ... 55 ° C. Dairy precipitation can be obtained at an electrolyte temperature of 55 ... 70 ° C and a current density of 20 ... 35 A / DM2. The microhardness of coatings from self-regulating electrolyte is 3000 ... 13 000 N / mm2.
The lack of such an electrolyte is a strong interaction of it with steel and other metals, resulting in retracting of the treated surfaces. Therefore, load parts into the bath only when the current is turned on. Anodes for chromening in self-regulating electrolyte It is recommended to apply from alloy: 90% lead and 10% GOST Tin. To prepare a self-regulating electrolyte, in the chromium bath dissolve the desired amount of chromium anhydride and fill water to the working level. Pre-chromic anhydride is analyzed on the content of sulfuric acid, which is removed from the electrolyte by adding carbon dioxide or strontium into it. 2.2 ... 2.3 g of carbon dioxide or 1.53 g of carbon dioxide are injected per 1 g of sulfuric acid. After the sulfuric acid deposition in the electrolyte, the desired amount of sulfate strontium and siliced \u200b\u200bpotassium is introduced and heated to a temperature of 50 ... 60 ° C. Heating lasts 15 ... 16 hours with periodic stirring every 2 ... 3 hours. After that, the electrolyte is ready for operation.
Adjust the electrolyte by systematic adding chrome anhydride. Along with chromium anhydride, carbon dioxide is introduced. Potassium silkmontluoride and sulfate strontium in the amount of 1 g / l are added when the surface of the extracted parts is approaching 1 m2.
Control of chromium coatings. In production conditions, the quality of coatings should be checked by external inspection and measurement of chromed surfaces. With an external inspection, it is necessary to pay attention to shine, deleniate and density of precipitate, uniformity and lack of peeling and other visible defects. Coating defects are obtained as a result of malfunctions in the work of chromium baths, for example, peeling the coating occurs as a result of insufficient degreasing and decaption, as well as in the presence of current breaks during the chromium process. Feling precipitation appears with insufficient contact details with suspension or at elevated current density. A non-uniform coating can be in the formation of a lead chromat film on the anodes, a lack of sulfuric acid, an excess of trivalent chromium. In order to avoid the above defects, it is necessary to adjust the electrolyte and eliminate other problems in the work of the chromium bath.
Equipment. The layout of the equipment for the restoration of parts of the chromium parts is shown in Figure 20.
Current sources - straighteners with voltage 12V VAKG-12 / 6-3000, Wagg-12 / 600m, you-600/300 and others, as well as low-voltage Anda generators 500/250, 750/375, 1000/500, 1500/750 . The baths for the electroplating area are made of sheet steel 4 ... 5 mm. Facing for washing and degreasing baths is not required. The inner surface of the chrome bath is lined with lead.
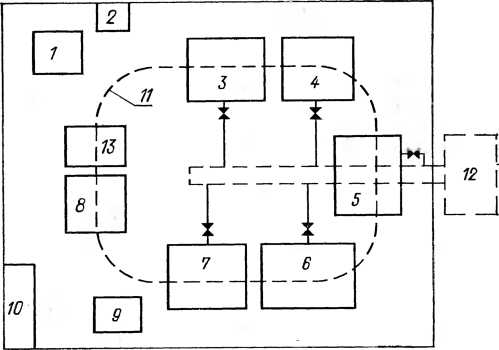
Fig. 20. Location of equipment
On the recovery site
Chrome details:
1 - rectifier; 2 - electrical panel;
3 - bath for electrochemical degreasing;
4 - bath for hot flushing;
5 - Bath for cold flushing;
6 - bath for decaption;
7 - bath for chromening;
8 - Bath for capturing electrolyte;
9 - Drying cabinet; 10- Racking Remfond;
11 - electrothelfer;
12 - collection-neutralizer;
13 - table for mounting and dismantling.
Materials. The estimated consumption of materials in grams of 1 dm2 of the reduced surface for the average thickness of the coating 0.1 mm during chromium in the universal electrolyte is given in Table 13.
The cost of restoration of 1 dm2 surface with chromium chromium in a universal electrolyte with a coating thickness of 0.1 mm approximately 44.8 kopecks., 0.2 mm - 52.0 kopecks., 0.3 mm - 58.6 kopecks.
Electrolytic iron has light gray, has a sufficiently high hardness and wear resistance. The chemical composition of electrolytic iron depends on the composition of the initial materials used in electrolysis.
In conventional precipitation using steel soluble anodes, the content of impurities in the coatings is within: 0.035 ... 0.06% C; 0.03 ... 0.05% s; 0.05 ... 0.01% p, 0.0009 ... 0.023% Si; up to 0.01% MP.
In electrolytic precipitation of iron, there are also impurities of such metals as Mg, Co, Ni and others, due to the content of these metals in the anodes and electrolytes. In addition, the electrolytic iron contains a significant amount of hydrogen highlighted on the cathode together with iron. Atomic weight of iron 55.85. Electrochemical equivalent 1.042 g / A-h.
Electrolyte compositions. At repair enterprises, hot chloride electrolytes consisting of two components were the greatest propagation for the magazine: iron and hydrochloric acid chloride. In repair practice, four types of electrolyte chloride are most often used, characterized by iron concentration.
The low-concentrated electrolyte contains 200 ... 250 g / l of iron chloride (FECL2-4H20). At a temperature of 60 ... 80 ° C and current density 30 ... 50 A / DM2, the electrolyte ensures the preparation of dense, smooth fine-grained precipitation of iron with a hardness of 4500 ... 6500 N / mm2, a thickness of 1.0 ... 1.5 mm. Iron yield over current is 85 ... 95%. The precipitation rate of iron is 0.4 ... 0.5 mm / h to the side. The electrolyte allows an acidity oscillation at electrolysis from 0.8 to 1.5 g / l, which is slightly reflected in the mechanical properties of coatings. The disadvantage of this electrolyte is a gradual increase in iron concentration in the process of electrolysis as a result of the inconsistency between the rate of dissolution of the anodes and the rate of iron precipitation on the cathode, which causes difficulties when maintaining an iron bath.
The average monitoring electrolyte of the optimal concentration contains 300 ... 350 g / l of iron chloride (FECL2-4H20). The cathode iron yield from this electrolyte at a temperature of 75 ° C and a current density of 40 A / DM2 is 96%. In that The electrolyte anodic and cathode outputs of iron for current becomes approximately the same, the concentration of iron remains almost unchanged and the electrolyte for a long time at the concentration of iron does not require adjustment. Currently, this electrolyte has been widely used at repair enterprises.
The midconditioned electrolyte contains 400 ... 450 g / l of iron chloride. The electrolyte is used to restore parts having sufficiently high wear and relatively low hardness. The electrolyte makes it possible to obtain smooth dense coatings with a thickness of up to 2 mm and the hardness of 2500 ... 4500 N / mm2. The electrolyte also finds the application to restore the seating holes in the housing, items.
The highly concentrated electrolyte contains 600 ... 680 g / l of iron chloride. The electrolyte at a temperature of 95 ... 105 ° C and current density 5 ... 20 A / DM2 allows you to get soft (120 ... 200 kg / mm2), viscous coatings with a thickness of 3 ... 5 mm ..
Recently, cold electrolytes have been developed that allow you to use higher current densities and ensuring high process performance.
Chloride Marganese MPS12-4N20 Ascorbic Acid Double-Terminal Iron FECL2-4H20 Chloride Marganese MPS12-4N20 Potassium Chloride KS1 (or) NaCl Ascorbic Acid Ballet Iron FECL2 * 4H20 sulfate iron FES04 * 7H20 Methyl sulfate iron FE (CH3OSO3) 2 * 4N20
Chloride electrolytes without additives, shown in Table * allow you to obtain high-quality wear-resistant coatings with a thickness of 0.6 ... 1.0 mm and to restore the wide range of worn items to normal performance and nominal sizes. The electrolyte, which includes a two-meter iron and iodide potassium, ensures the input of high-quality precipitation, iron 'subject to the use of asymmetric alternating current.
The presence of ascorbic acid in electrolytes allows electrolysis to conduct an electrolysis in a wide range of pH values \u200b\u200bfrom 1.8 to 6.0, which greatly simplifies the control of the acidity of the electrolyte. The electrolyte consisting of a two-meter iron and methyl sulfate iron compared with the chloride is less aggressive and more resistant to oxidation. Coatings obtained from this electrolyte have fewer cracks, possess a more uniform structure.
Preparation and adjustment of electrolyte. For the preparation of electrolyte chloride, a two-meter iron is used (Fe € L2-4H20).
Salonic acid (NS1) is used in the form of an aqueous solution of different concentrations with a density of 1.14 to 1,20. The preparation of the electrolyte is performed in the following order. The bath is poured a flow or distilled room temperature water and a hydrochloric acid is added at the rate of 0.5 g / l of water. In the acidified water, two-rod iron, withstanding the required concentration, and stirred to complete dissolution. After dissolving two-meter iron, the electrolyte must be standing for 1 ... 2 hours until the light green color arrives. The electrolyte is then tested for acidity. Normal acidity should be pH 0.8 ... 1.2. If necessary, add the missing amount of acid in accordance with its density below.
Acid density, g / cm3 1,14 1,15 1,16 1,17 1,18 1,19 1.20 Number of acid, g / l 20 19 18 17 16 15 14 Quantity of acid, cm * / l ...... 18 16.6 15.5 14.6 13.6 12.6 11.6
The electrolyte prepared in this way should be developed with a current at a density of 30 A / DM2 and the ratio of the surfaces of the anodes and cathodes of SA: Sk \u003d 2: 1 for two hours.
Specific electrolyte weight (density) g / cm8 1,12 1,15 1,17 1,20 1.23 1.26 1.29 1.32 1.35
Iron concentration, g / l ... 200 260 300 350 400 450 500 550 600.
The electrolyte acidity control can be carried out using the Ryfan's indicator paper with a pH of 0.3 ... 2.2 or LPU-01 potentiometers, LPM-60.